WebbWrites is committed to producing quality documents, including:
- Clinical study reports (Phase 1, 2, 3, and 4)
- Common Technical Document: Clinical Overview and Summaries for Modules 2.5 and 2.7
- Integrated Summaries of Effectiveness and Safety
- Clinical protocols
- Subject narratives
- Development Safety Update Reports
- Briefing Books for regulatory interactions
- Investigational New Drug applications
- Investigator’s Brochures and updates
- Manuscripts
- Quality assurance/quality control of documents
PROFESSIONALS COMMITTED TO PRODUCING QUALITY DOCUMENTS


Flexibility
Clinical study reports can typically be prepared within 2-4 weeks and summary documents within 4-6 weeks, depending on complexity. However, WebbWrites can accelerate document preparation using various methods, including finalization of non-data sections prior to data delivery, rolling receipt of data, rolling quality control of documents, use of draft data, real-time incorporation of comments during round-table reviews, and working on-site with the Sponsor.
Quality
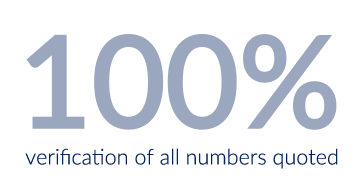
All documents undergo several internal quality-control checks prior to delivery, which consist of 100% verification of all numbers quoted in the text, as well as review by senior-level personnel for overall interpretation of the data.

Process
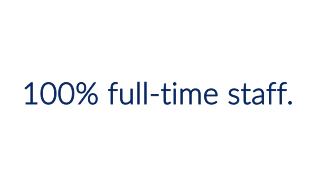
WebbWrites can provide a bid once foundation materials, proposed timelines, and any company-specific templates are provided. Sponsors work with the same WebbWrites’ point person throughout the life of their submission, as we do not subcontract and our employees are 100% full-time staff.
